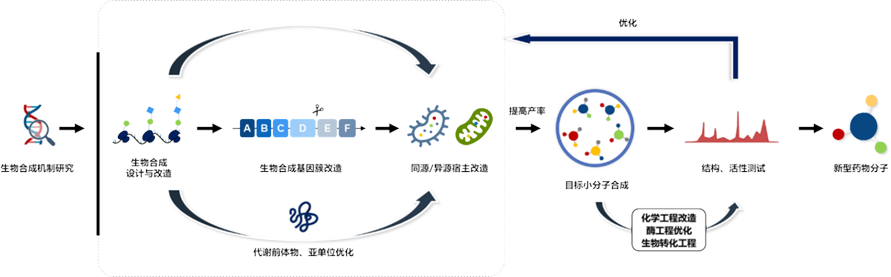
Synthetic Biology
Biostar is committed to the R&D of microbial metabolites innovative drugs, and has established advanced, unique and sustainable core technology platforms, including combinatorial biosynthesis, microbial fermentation production, microbial drug formulation development platforms.

Combinatorial Biosynthesis Platform
Based on the combined biosynthesis platform, the we in-depth study the microbial synthesis mechanism of candidate compounds with potential druggability, design the molecular structure of drugs, and directionally transform the microbial metabolite synthesis process to obtain target compounds with industrialization potential.
Microbial Fermentation Production Platform
Based on the microbial fermentation production platform, we design the fermentation process, including the configuration of media and the research of COAs, to successfully realize the transformation of target compounds from the pilot stage to large-scale production.


Microbial Drug Formulation Development Platforms
Based on the microbial drug formulation development platforms, we solve the bottleneck of microbial drug formulation, develop a variety of microbial drug dosage forms, and further improve the product efficacy and safety, as well as enhance patient compliance.